I read trade publications that cover everything from banking to biotech, looking for interesting perspectives on data analysis and statistics, especially where it pertains to quality improvement.
Recently I read a great blog post from Tony Taylor, an analytical chemist with a background in pharmaceuticals. In it, he discusses the implications of the FDA's updated guidance for industry analytical procedures and methods validation. His audience comprises analytical chemists and pharmaceutical researchers, people who are very technologically savvy and adept at solving problems. The kind of people you'd imagine are very capable and eager to collect some data and figure out what it means.
Or maybe not.
What Taylor's post makes clear is that even a highly educated, scientifically inclined audience like this doesn't necessarily appreciate the value of statistical analysis—or at least, doesn't really enjoy actually doing it.
Taylor acknowledges an issue that Minitab has focused on from its earliest days: when it comes to analyzing data and using statistics, some people seem to get it right away, and others don't. Some people enjoy it, and many others find it tedious or even painful.
Those who fall into that second category—even highly educated analytical chemists—tend to try to avoid statistics, even though there are many tools available to help them get the benefits of analyzing their data more easily and quickly. The problem is that trying to avoid statistics and data in today's world is like an ostrich burying its head in the sand so it won't see a threat.
Taylor points out that new FDA guidelines make this particularly true in the pharmaceutical realm, where "the use of statistics will only be increasing in the future."
So does this mean every analytical chemist need to be a statistician? No. But Taylor makes a strong case that good analytical chemists at least need to appreciate and be prepared to apply statistical methods in their work—and that's excellent advice for people in most professions.
Tools that Make Statistics and Data Analysis Easier
Minitab has always sought to help more people understand and apply statistics, so it's extremely gratifying that Taylor gives us a shout-out by name:
If one wishes to utilize some of the most useful aspects of statistical experimental design and optimization, then one would need to use a simple statistical tool such as Minitab®...This program is very easy to use, and with only a rudimentary understanding of the principles, can help us to improve our analytical practice enormously.
Now that's music to our ears! Our goal has always been to make Minitab easy to use, and by adding features like the Assistant to guide you through statistical analysis and help you interpret the results correctly, we are striving to open the world of data analysis to as many people as possible.
Taylor goes on to call out Minitab's design of experiment (DOE) capabilities, which certainly have great application in chemistry and pharmaceutical research. He notes that with Minitab:
...it is very straightforward to plan, implement, and analyse Fractional Factorial designs which allow us to investigate the dominant variables within a method and help to control them more effectively. We can use the same methods for identifying interactions between variables in our methods and control or eliminate them. It is very easy to plan a fractional factorial design such as the Plackett-Burman, in order to significantly reduce the number of experiments required to validate an analytical method for Robustness. We can also use full factorial methods to optimise an analytical method in significantly fewer experiments using the tools available within Minitab.
Though Taylor doesn't mention it specifically, one of my favorite tools in Minitab is the DOE Assistant. Designed experiments are extremely powerful, but there is a perception that they are difficult to set up and analyze. The Assistant will actually guide you through the process of designing and analyzing both screening and optimization experiments step by step, and even gives you output that puts your results into straightforward language that's easy to understand and share with others, regardless of their level of expertise.
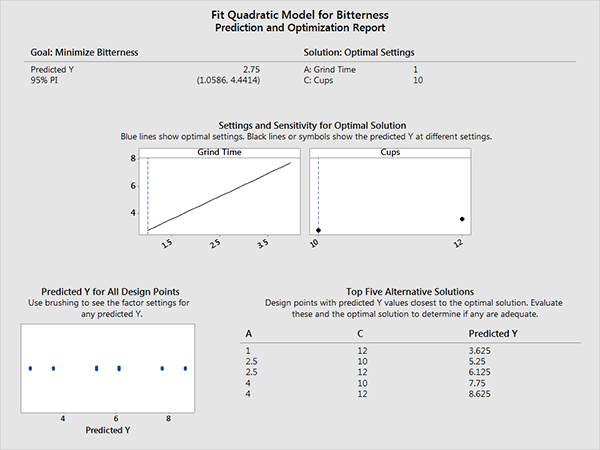
A DOE optimization report created with the Assistant in Minitab 17.
Resources for Using Statistics in Pharmaceuticals
If you're just getting started with statistics and/or Minitab, check out our e-learning course, Quality Trainer. You can also download Minitab Statistical Software and try it free for 30 days.
We've also developed an instructor-led training program that can help you use statistical methods to validate a pharmaceutical process for each stage of the FDA Process Validation Guideline.
If you're using Minitab Statistical Software, we offer resources to help with your validation, including Minitab’s software validation kit here:
http://www.minitab.com/support/software-validation/
This software validation kit was created to help you understand how we validate Minitab Statistical Software for market readiness.
Finally, Minitab's statistical consultants—highly respected statisticians with experience in pharmaceuticals, medical devices, and many other industries—can help you overcome even the toughest data analysis challenges.
Whatever industry you're in, wherever you are on the continuum of statistical experience and expertise, we encourage you to get the maximum benefit from the data you're collecting and analyzing, and we'd love to help.



