What's the last thing you ever want to be in your food? Pathogens. These tiny bacteria, viruses and microorganisms can cause disease and infection for anyone who comes in contact with them. It's especially vital for manufacturers in the food and beverage industry to ensure food safety in their products, so their consumers don't get sick.
Nothing in life is perfect though; 100% prevention is nearly impossible. If and when food safety is compromised, a Failure Modes and Effects Analysis (FMEA) can be used to help.
FMEA: What Is It?
FMEA is a tool that can help systematically organize, assess and prioritize failures based on their severity. Once failures are compiled and ranked, an FMEA can also help with developing a plan of action for addressing failures if they do occur and preventing them if possible.
There are a variety of FMEAs available; all of them focus on risk assessment, identification, quantification and mitigation of failures and issues. The two types organizations use the most are:
- DFMEA: Design Failure Modes and Effects Analysis, which looks at the issues with the product, including concept and design.
- PFMEA: Process Failure Modes and Effects Analysis, which focuses on the risks in the process rather than the product itself.
Using COA and FMEA to Maintain High Quality Food Standards
Food manufacturers often have protocol in place to ensure that the ingredients they receive arrive with a Certificate of Analysis (COA). The supplier provides this certificate to the food manufacturer buying the ingredient to indicate it has passed their tests for quality and purity (including that an acceptably low level of pathogens were detected). Food manufacturers can just trust the COA from their suppliers and hope everything is exactly to their standards, or use an FMEA to validate the COA and have full confidence in their products.
Production processes can also expose food or beverages to pathogens or other food safety issues. This is where a PFMEA is the perfect tool to really study that process and find where the failure is occurring, but where you do begin?
Completing a Failure Mode and Effects Analysis
Minitab Workspace makes it easy to complete any FMEA, whether it's design or process focused. Once finished, it's a breeze to export and share any FMEAs you've created with the rest of your team and organization to keep everyone on the same page, get feedback and execute any changes needed.
A typical FMEA usually involves these steps:
- Identify potential failure types, or "modes," for each step of your process or design concept.
- List the effects that result when with those failures occur.
- Identify potential causes for each failure mode.
- Record existing controls that are in place to keep these failures from happening.
- Rate the Severity of the effect, the likelihood of Occurrence, and the odds of Detecting the failure mode before it causes harm.
- Multiply the values for severity, occurrence, and detection to get a risk priority number (RPN).
- Improve items with a high RPN, record the actions you've taken, then revise the RPN.
Start Your Own FMEA and Access Over +90 Tools

Below is an example of a PFMEA Form in Minitab Workspace:
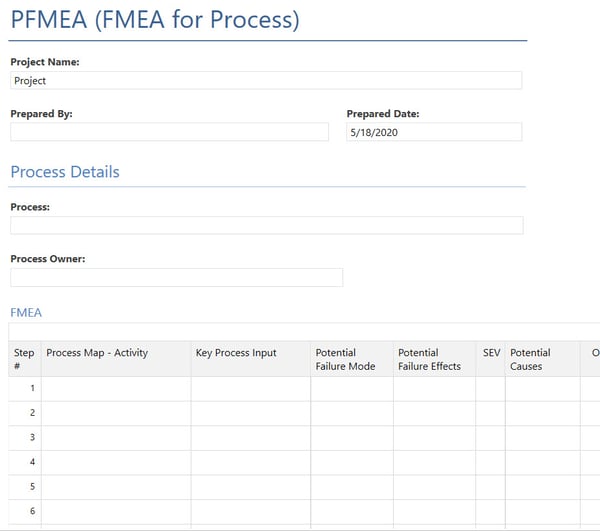
By creating and keeping your FMEA in Minitab Workspace, you allow it to be a living document that can be updated as needed.
Once you've filled in the top few fields, you can begin entering information in the form table. Here's some guidance to get you started:
- Process Map - Activity: Enter each step in the process, feature or type of activity. If a food manufacturer were baking cupcakes, example steps would include cracking the eggs, making the cupcake mixture and creating the frosting to name a few.
- Key Process Input: Write in the key components or inputs of each step listed under Process Map - Activity.
- Potential Failure Mode: Add the ways the process can fail for each activity. Remember, there may be many ways it could fail. In the cupcake example, eggs being kept at the wrong temperature or using eggs that are already cracked would be a potential failure mode.
- Potential Failure Effects: Detail the possible fallout of each type of failure without forgetting there may be multiple failure effects. In the cupcake manufacturer example, eggs kept at the wrong temperature would lead to the waste of raw materials and might lead to undetected pathogens and unsafe products.
See how to finish completing your FMEA here or reach out to our best-in-class Minitab Support with specific questions.
Feeling Confident with an FMEA
Once you've completed an FMEA, you'll have answers to the questions below and feel even more confident in the quality of your product.
- What are the potential failure modes at each step of a process?
- What is the potential effect of each failure mode on the process output, and how severe is it?
- What are the potential causes of each failure mode, and how often do they occur?
- How well can you detect a cause before it creates a failure mode and effect?
- How can you assign a risk value to a process step, that factors in the frequency of the cause, the severity of failure, and the capability of detecting it in advance?
- What part of the process should an improvement project focus on?
- Which inputs are vital to the process, and which aren't?
- How can reaction plans be documented as part of process control?




